Attorney General Nick Brown joined 19 state attorneys general in a joint statement affirming the safety of medication abortion drug mifepristone as the FDA reviews access following pressure from Republican state attorneys general seeking restrictions or withdrawal.
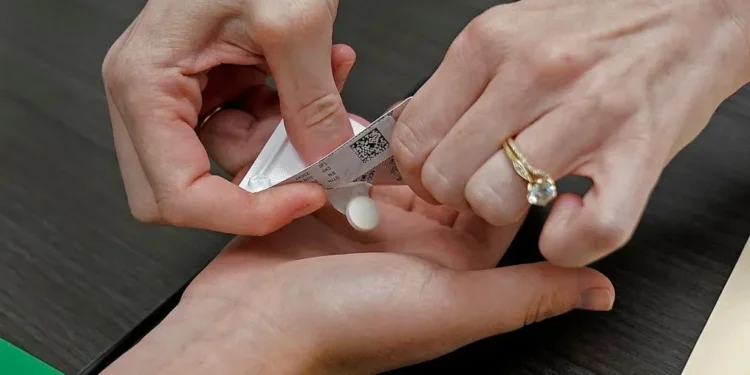
The statement emphasized mifepristone’s 25-year safety record in the United States and globally, noting it represents the most common method for early-term abortion care and serves as standard treatment for managing early miscarriage.
The attorneys general criticized the FDA review as responding to a “scientifically baseless letter” that ignores decades of research demonstrating the drug’s safety and efficacy. “Medical decisions should be left between patients, their families, and their providers, and they should be guided by science, not political agendas,” the statement declared.
The coalition pledged enforcement action if mifepristone access faces challenges, asserting their responsibility to enforce state laws and protect residents’ reproductive healthcare access.
Mifepristone received FDA approval in 2000 for use with misoprostol as the only FDA-approved regimen for ending early pregnancy. Hundreds of scientific studies support medication abortion safety and efficacy, with more than 7.5 million American women having safely used mifepristone for abortion care or miscarriage management.
Washington State Department of Health data indicates that among nearly 30,000 medication abortions provided in Washington during 2023 and 2024, fewer than 0.2% resulted in complications severe enough to require hospitalization.
The statement signatories include attorneys general from Arizona, California, Colorado, Connecticut, Delaware, District of Columbia, Hawaii, Illinois, Maine, Maryland, Massachusetts, Michigan, Minnesota, New Jersey, New Mexico, New York, Oregon, Rhode Island, Vermont, and Washington.
The FDA review follows ongoing national debates about abortion access following the Supreme Court’s Dobbs decision overturning Roe v. Wade. States have adopted divergent approaches to abortion regulation, with some implementing restrictive laws while others have expanded access and protections.
Mifepristone’s availability has become a central battleground in reproductive rights disputes, with supporters citing extensive safety data while opponents seek FDA restrictions despite scientific evidence.
The multi-state coalition’s statement represents coordinated resistance to potential federal restrictions on medication abortion access in states that have maintained or expanded abortion rights following the Dobbs decision.