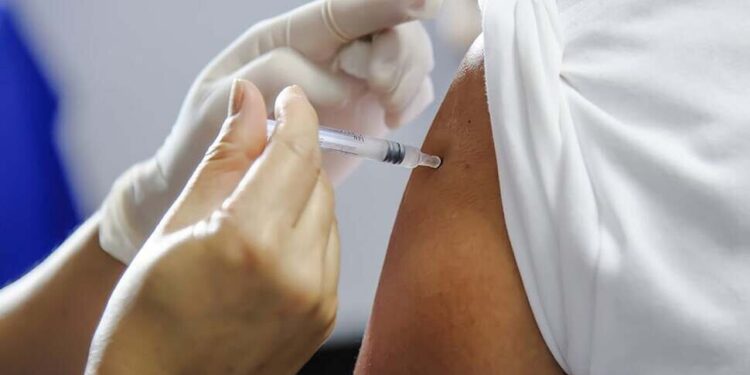
The FDA refused to review Moderna’s application for an mRNA-based flu vaccine, a move that experts warn could discourage research into the technology that enabled rapid COVID-19 vaccine development during the pandemic.
Moderna said it’s requesting a meeting with the FDA after receiving a “refusal-to-file” letter, meaning the agency won’t review the company’s advanced-stage clinical trials. A review would be the next step toward licensed approval. Moderna and Pfizer-BioNTech already produce mRNA-based COVID vaccines, but no licensed mRNA flu vaccine exists on the market.
Dr. Seth Berkley, a senior advisor to the Brown University Pandemic Center, called the letter “relatively unusual” from the FDA. Moderna had meetings with the FDA before launching its Phase 3 study and received approval to proceed. Moderna published study results showing the mRNA flu vaccine had higher efficacy than a traditional flu vaccine with a good safety profile. The FDA could have asked for additional information rather than refusing review entirely. “But the fact that they just said we’re not going to review it is a little bit of a bait and switch,” Berkley said.

Berkley called this refusal “worrisome” and said it could further chill vaccine research, including mRNA studies. Companies won’t invest tens of millions if they expect stonewalling during FDA consideration. “It’s ideology, not science that’s doing this,” he said. Health and Human Services, under Robert F. Kennedy Jr., has already pulled about $500 million in government funding for mRNA vaccine development and stopped funding for Moderna’s bird flu mRNA vaccine testing.
HHS Spokesman Andrew Nixon said the FDA rejected Moderna’s application because “the company refused to follow very clear FDA guidance from 2024 to test its product in a clinical trial against a CDC-recommended flu vaccine to compare safety and efficacy.” Nixon said Moderna exposed study participants 65 and over to increased risk by giving them “substandard care” against FDA scientists’ recommendations. Moderna CEO Stéphane Bancel said the decision “does not further our shared goal of enhancing America’s leadership in developing innovative medicines.”
Berkley said Moderna’s Phase 3 trials included around 44,000 participants, enough to demonstrate safety and efficacy. He noted Moderna offered results from a separate trial comparing the mRNA vaccine against a high-dose shot. Berkley said an mRNA flu vaccine could theoretically be more responsive to dominant virus strains due to faster development compared to traditional flu vaccines, noting mRNA technology produced a COVID vaccine in 11 months versus the previous record of four years.